Background: Novel therapies for patients with relapsed/refractory acute myeloid leukemia (R/R AML) are imperative, particularly those who have experienced relapse or resistance to multiline chemotherapies or venetoclax-based regimens, thereby underscoring the need for more innovative therapies that are both effective and well-tolerated. Chidamide and Selinexor have shown potential synergistic anti-leukemia effects by targeting NF-Κb /c-FLIP signaling via suppressing HDAC1/XPO1 activity. Therefore, the aim of this study was to investigate the activity and tolerability of chidamide combined with selinexor regimen for R/R AML.
Methods: We report the findings of a phase 2 study in patients with relapsed/refractory AML that combined chidamide with selinexor. Selinexor was administered orally twice weekly for 4 weeks at a dose of 40/60 mg, depending on the patent's weight (over or under 70 kg). Chidamide was taken orally once a day at a dose of 10mg for 28 days. The primary endpoint was composite complete remission rate [CRc, complete response (CR) plus complete response with incomplete blood count recovery (CRi)] after 1 cycles of treatment. The secondary endpoints include safety and survival.
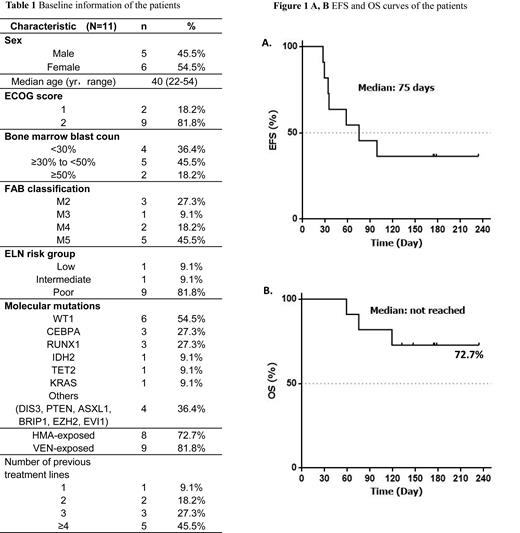
Results: Between December 2022 and April 2023, a total of 11 patients, median age 40 years (range, 22-54) were enrolled in the trail (ClinicalTrials.gov number, NCT05951855.). 8 out of 11 (72.7%) patients with R/R AML had received more than three prior lines of treatment. All of patients were previously exposed to venetoclax or hypomethylating agents (HMAs). Key baseline and clinical characteristics are summarized in Table 1. 10(90.9%) patients received the initial dose of 40 mg of selinexor twice a week, while one (9.1%) received 60 mg. Chidamide was administered to all patients at a daily dose of 10 mg daily. Five (45.5%) patients achieved a CRc (with 3 CR and 2 CRi), two (18.1%) achieved Partial remission (PR), and 5 (45.5%) had progressive disease for an overall response rate (ORR) of 63.6%. With a median follow-up of 147 days(range 59-234), the median event-free survival (EFS) was 75 days and median overall survival (OS) was not yet reached (Figure 1). Among the seven patients who achieved CRc or PR, all proceeded to transplant after responding. One relapsed on day 56 following response and died 20 days later. Another patient died from an infection during the allogeneic hematopoietic stem cell transplantation (allo-HSCT). The remaining 5 patients are currently alive as of July 2023. No dose-limiting toxicities were observed. Common adverse events included neutropenia (72.7%), thrombocytopenia (63.6%), nausea/vomiting (63.6%), fatigue (45.5%), diarrhea (18.1%), bacteremia (9%).
Conclusion:
In this study, the combination of Selinexor and chidamide was administered as salvage treatment for R/R AML patients, resulting a high response rate and favorable safety profile. This offers a potential way for patients to bridge to transplantation. However, future studies with larger sample sizes and longer follow-up periods are needed to further validate its efficacy.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Selinexor (KPT-330) is a small molecule, oral, first-in-class, potent selective inhibitor of nuclear export (SINE) compound. Selinexor has received approval from the US Food and Drug Administration for RRMM patients and relapsed/refractory diffuse large B-cell lymphoma patients. Several studies have confirmed that selinexor monotherapy or combination therapy has good efficacy and good tolerance for AML patients. Chidamide is a novel benzamide class of histone deacetylase (HDAC) inhibitor (HDACi) that was approved by the Chinese Food and Drug Administration in 2014 for the treatment of relapsed or refractory (R/R) PTCL patients. But it shows promise in leukemia. In a phase 1/2 study, Chidamide was administered in relapsed/refractory AML combined with decitabine, cytarabine, aclarubicin, and granulocyte colonystimulating factor (DCAG). A total of 93 patients with r/r AML were enrolled. Overall, 24 patients had a complete remission (CR) and 19 patients achieved CR with incomplete blood count recovery (CRi). The overall response rate (ORR) was 46.2%. The regimen showed good antileukemic activity and acceptable toxicity.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal